Corrosion Lab
Corrosion (Task 1)
By adding chemical indicators to the solution in which the metals are placed allows the change of colour when certain ions are present. One of the chemical indicators which were used was potassium Ferricyanide. When Fe2+ ions were present this turned blue. The other indicator which was used was Phenol Phthalein. This indicator turned red in the presence of OH– ions. This means that if there was any blue in colour this confirms the anode and the presence of iron and if it was red it confirms the side of the cathode because of the presence of hydroxyl.
In terms of anodes and cathodes the least noble metal of the two will act as the anode and corrode.
When the Anode Corrodes
Experiment
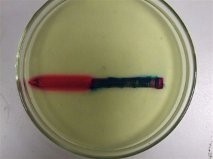
Mild Steel Nail Partially Plated with Copper
Below shows the Mild Steel nail which had been partially plated with copper at the pointed end and placed into the solution. Because the copper end has turned red this shows that it is acting as the cathode so will not be corroding. Because the mild steel is less noble than the copper it is acting as the anode and therefore corrosion is occurring.. Also on the pointed end of the nail it shows to have traces of blue which suggests that this is also acting as an anode.
Above: Mild Steel Nail Partially Plated with Copper
Mild Steel Nail Connected to Zinc Strip
Below shows the Mild Steel nail which has been connected to the zinc strip. It shows the Mild Steel nail turning red which shows it is acting as the cathode. The zinc part is acting as the anode but there is no blue colour because of the lack of iron present in it. The reason the zinc is corroding is because it is less noble than the Mild Steel.
Above: Mild Steel Nail Connected to Zinc Strip
Deformed Mild Steel Nail
Below shows a Mild Steel Nail which has been deformed. The point of where the nail was deformed has turned blue which shows that this area is acting as the anode. The other part of the nail which had turned red is acting as the cathode. Distressted areas within the metal are most likely to corrode first which is the case in this picture with the bend, pointed end and head all turning blue.
Above: Deformed Mild Steel Nail
Mild Steel Nail Partially Plated with Zinc
Below shows a Mild Steel nail which has been partially plated with zinc on the pointed end. The mild steel part has turned red which shows this is the area acting as the cathode. This means the Zinc is acting as the anode, but because there is no iron present it will not turn blue around the zinc. The reason that the zinc is acting as the cathode is because it is a less noble metal than the mild steel.
Above: Mild Steel nail plated with Zinc
Mild Steel Nail
Below shows a mild steel nail which has been completetly corroded. This is because the Mild steel is acting as both the anode and the cathode. Down the length of the nail shows a little pink colour because this area is indicating mainly a cathodic reaction, where the pointed end and head show more blue because these are the weaker points which will lean towards an anodic reaction, these areas will corrode quicker than the rest of the nail.
Above: Mild Steel nail
Mild Steel Nail Partially Plated with Copper Connected to a Zinc Strip
Below shows a Mild Steel nail partially plated with Copper connected to a Zinc strip. Because the nail is completely pink it shows that all of the nail is acting as the cathode while the zinc strip is acting as the sacrificial anode to protect the copper and steel. This is because out of all three metals, the zinc is the least noble.
Above: Mild Steel Nail Partially Plated with Copper Connected to a Zinc Strip
Droplet
Above: A diagram to show the corrosion which occurs in the droplet (http://www.gordonengland.co.uk/xcorrosion.htm, last accessed April 2014)
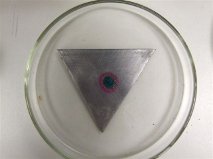
Below is the sample with the drop containing 3% sodium chloride, 5% potassium ferri-cyanide solution and 1% phthalein solution on the mild steel plate. The centre of the sample has turned blue which shows that it is acting as the anode. This is because of the formation of Fe2+ ions have formed because of the corrosion of the steel. When the phenol phthalein solution turns pink it shows that hydroxyl ions are present and is acting as the cathode. This can be seen on the picture around the edges of the blue part. The reason for this is because of the levels of oxygen which is available to corrode the sample. The middle of the drop has further for the oxygen to diffuse through to get to the metal, whereas on the edges there is less to diffuse through so more oxygen is available in order to corrode the sample to produce the OH– ions. The orange ring is a product of the Fe2+ ions and the OH– ions reacting with each other to produce rust.
Above: Shows a Mild steel plate which has a drop of solution in the middle.
Task 2
Sample 1
Below is a picture of a brass radiator which has been cut open to show the internals of it. It shows small dots within the inside of it. This has because it has undergone dezincification, a process in which the zinc separates from the brass. The process takes place by firstly the brass dissolving. When this brass dissolves the zinc ions stay in solution and the copper plates back on. It can precede in the absence of oxygen, however the presence of oxygen increases the rate of the attack. Analysis of de-zincified areas usually shows 90-95% copper with some of it present as copper oxide. Slightly acidic water, low in salt content and at room temperature is likely to produce a uniform attack. Whereas natural or alkaline water, high in salt content and above room temperature often produces plug type attack. In order to help reduce the corrosion on the radiator, reducing the oxygen in the system along with using slightly acidic water could help slow it down. Using other materials such as Duplex can also increase the corrosion properties of the radiator as I industry this material is used in heat exchangers which are similar. However using materials like this would increase the cost for the component.
Above (Fig , showing the internals of a brass radiator)
Sample 2
Below is a picture of a steel component which has undergone corrosion when being used in elevated temperatures in corrosive atmospheres. High temperatures greatly increase the rates of corrosion which occurs on a component. Also increasing the concentreation of H+ ions in acids. A certain amount of erosion corrosion will occur in this component because of the corrosive atmospheres that are passive through it. This is caused by chemical attack along with the motion of these chemicals passing by it. It appears that the component itself has been used as some type of exhaust for fumes, This means that there are corrosive fumes that will be attacking the component to corrode it. Added to an elevated temperature and this corrosion is increased. The component is corroded evenly all the way round it so suggests it has undergone uniform attack as opposed to being localised such as pitting. In order to help reduce this type of corrosion a different material could be used such as Inconel which has very good corrosion properties at high temperatures. Using materials like this would increase the cost so calculations would need to be carried out to make sure the extra cost increases the lifespan enough to make it viable to use. Inhibitors could also be added to the corrosive environment. These are substances that are added to the corrosive environment to decrease its corrosiveness.
(Pages 692-698, Materials, Science and Engineering, William D Callister-David G. Rethwisch, 2011)
References
http://www.gordonengland.co.uk/xcorrosion.htm, last accessed April 2014
Materials, Science and Engineering, William D Callister-David G. Rethwisch, 2011